Long-term Follow-up of Suspected Vaccine-Induced Papillitis: A Teaching Case Report
Brianne Hobbs, OD, FAAO, and Kaila Osmotherly, OD, FAAO
Abstract Papillitis is a condition that necessitates prompt diagnosis and treatment in the pediatric population. Pertinent differentials should be considered and excluded through imaging and appropriate lab tests to rule out any treatable conditions. Papillitis in children most commonly presents following an infection but has been reported to occur after vaccination. These patients often develop optic atrophy as a result of the preceding inflammation. However, few studies address the long-term follow-up of pediatric patients with papillitis.
Key Words: papillitis, optic neuritis, vaccination, optic atrophy, OCT
Background
Optic neuritis results when the optic nerve becomes inflamed. Two major clinical presentations of optic neuritis exist: typical demyelinating and atypical. Typical demyelinating optic neuritis is the most common presentation and includes the following features: acute monocular vision loss (which typically improves after two weeks), pain on eye movement, visual field defect and relative afferent pupillary defect (RAPD) on the affected side.1 Demyelination in typical optic neuritis may be idiopathic or induced by a systemic demyelinating disorder. Atypical optic neuritis is characterized by the following features: occurrence in a patient under age 14 or over age 45, bilateral presentation, vertical hemianopic visual field defect, recent sinusitis and/or progression of the optic neuritis for more than two weeks.1 Atypical optic neuritis is more concerning than typical demyelinating optic neuritis and indicates the need to perform additional testing for systemic conditions.
Optic neuritis is also classified into two principal types according to what portion of the optic nerve is involved.1-3 Papillitis, also known as anterior optic neuritis, occurs when the intraocular portion of the optic nerve is inflamed. Retrobulbar optic neuritis affects the segment of the optic nerve posterior to the globe.1-3 Papillitis is more common in children than adults.1-3
The etiologies of optic neuritis may be classified into four categories: demyelinating, infectious, non-infectious and parainfectious.2 The axons of the retinal ganglion cells become myelinated after passing through the lamina cribosa, and demyelination occurs when the myelin is phagocytosed by the microglia and macrophages, thus interrupting axonal conduction.2 Demyelinating optic neuritis may be the first sign of multiple sclerosis (MS). Neuromyelitis optica, another systemic demyelinating disease, is characterized by bilateral optic neuritis in addition to demyelination of the spinal cord. Infectious etiologies of optic neuritis include, but are not limited to, sinusitis, cat-scratch fever, syphilis and Lyme disease.2 Non-infectious causes of optic neuritis include sarcoid, systemic lupus erythematosus and polyarteritis nodosa.3 Parainfectious causes of optic neuritis include immunizations and viral infections such as chickenpox, measles and rubella.2 Despite reduced visual acuity in the acute phase, most patients regain their prior visual acuity, even without treatment. Long-term complications of optic neuritis may include optic nerve atrophy, reduced visual acuity, decreased contrast sensitivity and reduced color vision.3
This case report focuses on the etiology, clinical presentation and prognosis of optic neuritis in the pediatric population and specifically investigates a rare cause of papillitis — vaccines. This report also addresses the potential long-term sequelae of papillitis in children. This teaching case report would be appropriate for third- and fourth-year optometry students as well as optometric residents.
Case Description
Initial presentation to outside provider in 2004 (records were obtained with patient’s permission)
 Figure 1A. During routine examination, C/D ratio in the right eye was noted to be .7/.7. |
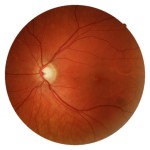 Figure 1B. During routine examination, C/D ratio in the left eye was |
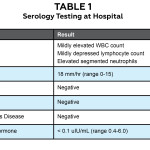
A healthy 12-year-old patient presented with a complaint of vision loss in his right eye upon awakening. Specifically he noticed that a portion of his face was obscured by blur when he looked in the mirror and that “everything had a red hue to it.” Associated symptoms included mild pain upon eye movement. He had noticed a headache, which was relieved with Tylenol, two days prior to the onset of vision loss. Visual acuity was 20/400 OD and 20/20 OS. His ocular history was notable for myopia. The patient was of normal weight; his medical history was remarkable for motion sickness. He had received vaccinations for hepatitis B and tetanus approximately four weeks earlier. Family history was positive for thyroid disease. Pupil evaluation revealed a 3+ RAPD in the right eye in association with a visual scotoma on visual field testing in the same eye; the left eye showed no visual field defect. A dilated fundus exam revealed optic nerves judged to be .2/.2 right eye and .3/.3 left eye with no apparent edema or pallor. The patient was referred to the emergency department for additional evaluation.

Figure 2. OCT showed retinal nerve fiber layer thinning in all quadrants and optic nerve atrophy.
Click to enlarge
 Figures 3A and 3B. Threshold visual field testing showed minimal diffuse loss on the mean deviation and no diffuse loss on the pattern deviation, indicating that the patient did not have functional vision loss. |
A head CT without contrast was obtained and the results were interpreted as normal. A T1/T2 weighted MRI of the brain, orbits, face and neck with and without contrast and fat saturation were obtained later that day, but no abnormalities were evident.
The patient was diagnosed with a complicated migraine following this visit. The assessment note stated: “The patient’s history and findings are most consistent with that of a migraine headache with a neurologic deficit related to the retina and/or optic nerve.” The patient was subsequently discharged and no treatment was prescribed.
Five days later, the patient was admitted to the hospital due to acute vision loss in his left eye. The patient noted slight pain on eye movement at this time in addition to the vision loss now present in both eyes. His visual acuity was finger counting OD and 20/400 OS. A 2+ RAPD OD was noted. Extraocular motility was full, but visual fields showed restriction in the superior field of the right eye with a full confrontation visual field in the left eye. The patient was dilated in the hospital and both nerves showed evidence of mild swelling in the absence of hemorrhages or exudates. Serology was obtained (Table 1). The diagnosis of bilateral optic neuritis secondary to hepatitis B vaccination was made at this time, and the patient began intravenous methylprednisolone 250 mg every six hours for three days. His vision gradually returned and was considered normal 12 days after he experienced his initial symptoms.
Present-day examination (2014)
The patient presented to the Midwestern University Eye Institute for a routine eye examination. He reported a history of optic neuritis secondary to hepatitis vaccination when he was 12. His ocular history was otherwise unremarkable. His corrected visual acuities were 20/15 OD, OS, and all other entrance testing was normal. No afferent pupillary defect was noted. Red cap testing was equivalent in each eye. Color vision was normal OD, OS with both D-15 and desaturated D-15 color tests. Contrast sensitivity testing was performed using the Pelli-Robson contrast sensitivity test and was assessed at 1.95 OD, OS. Intraocular pressures were 17 mmHg OD, OS. Upon dilation, his optic nerves appeared to have mild diffuse pallor and were suspicious for excavation. The C/D ratio was judged as .7/.7 OD, .65/.65 OS (Figures 1A and 1B). Retinal nerve fiber layer (RNFL) thinning was present in all quadrants on OCT (Figure 2). Threshold visual field testing (Figures 3A and 3B) revealed non-specific diffuse loss.
Education Guidelines
Discussion points
1. What is the pathophysiology of optic neuropathy?
2. What are the two major anatomic classifications of optic neuritis?
3. What is the most common type of optic neuritis in adults?
4. What is the importance of myelin in axonal conduction relative to optic neuritis?
5. What are the characteristics of atypical optic neuritis?
6. What are causes of compressive optic neuropathy?
7. What are the differentials for acute unilateral vision loss in children?
8. What laboratory tests would be appropriate for a child presenting with a swollen optic nerve?
9. What are the differentials for optic nerve pallor in a young adult?
10. How do the two major presentations of optic neuritis differ anatomically?
11. What symptoms are most common in adults presenting with optic neuritis?
12. What symptoms are most common in children?
13. What are the most common causes of optic neuritis in an adult population?
14. What are the most common causes in a pediatric population?
15. What clinical tests can be utilized to confirm the diagnosis of optic neuritis?
16. What are appropriate treatment options for optic neuritis?
17. Are there any trials that provide strong evidence for specific treatments?
18. What were the inclusion criteria in these studies?
19. What are the long-term consequences of optic neuritis?
20. Can these consequences be prevented?
Learning objectives
1. Define optic neuropathy and list the major categories of optic neuropathy
2. Review the major causes of optic neuritis
a. demyelinating
b. infectious
c. non-infectious
d. parainfectious
3. Understand the anatomical variants of optic neuritis and review the four anatomical divisions of the optic nerve as it travels to the brain
a. retrobulbar vs. papillitis
b. intraocular
c. intraorbital
d. intracanalicular
e. intracranial
4. Recognize the characteristics that make an optic neuritis atypical
a. understand the implications of atypical optic neuritis
5. Discuss the most common symptoms associated with optic neuritis
6. Discuss the most common signs associated with optic neuritis
a. identify clinical tests that aid in the diagnosis of optic neuritis
7. Identify the signs and symptoms necessary to make a clinical diagnosis of optic neuropathy
a. presentation
b. course of the illness
8. Identify the differences in optic neuritis presenting in adults and children in regard to the following
a. symptoms
b. ocular signs
c. associated conditions/etiology
d. natural history/prognosis
9. Understand the proper treatment for optic neuritis and when treatment should be utilized
10. Identify the long-term complications of optic neuritis
11. Develop the knowledge to counsel patients on the likely outcome of optic neuritis
Key concepts
- Visual acuity is not the most sensitive measure of visual function in patients with optic neuritis; therefore, additional characteristics such as contrast sensitivity should be assessed.
- The optic nerve head is more likely to be swollen in pediatric optic neuritis than in adult optic neuritis, and the presentation is often bilateral in children.
- The most common etiology of pediatric papillitis is a preceding viral illness (parainfectious).
- Optic neuritis has a favorable prognosis for complete visual recovery in children and is less likely to be predictive of MS than optic neuritis occurring in adults.
- A patient with a history of pediatric optic neuritis should be followed for the development of optic atrophy and can be monitored with visual field testing and OCT.
- Although most patients exhibit some degree of optic atrophy following optic neuritis, visual acuity is not always reduced.
Discussion
Differential diagnosis for optic neuropathies in children pertinent to this patient’s history
1. Complicated migraine
The diagnosis of complicated migraine was the patient’s initial diagnosis, which was made in the ER. This diagnosis was likely made based on the patient’s symptoms and normal imaging. The treating physician may have been referring to a retinal migraine or an ophthalmoplegic migraine. Retinal migraines can present with unilateral vision loss and visual field defects; the vision loss is typically transient (lasting less than one hour) and either precedes or presents concurrently with the headache.4 This diagnosis is inconsistent with the patient’s presentation as his headache preceded visual symptoms by two days. Retinal migraines are considered to be a diagnosis of exclusion,5 and at the initial presentation not all differentials had been ruled out. The term “complicated migraine” was historically used to describe an ophthalmoplegic migraine (a headache associated with oculomotor nerve palsies).5 This patient did present with an RAPD of the right eye, but did not demonstrate any other oculomotor deficit. Although this was the initial diagnosis made by the ER physician, given the patient’s presentation, it was not the most appropriate diagnosis.
2. Compressive optic neuropathy
Compressive optic neuropathy is due to a mechanical force acting on the nerve caused by a space-occupying lesion or enlarged extraocular muscles secondary to thyroid eye disease. This patient did not have thyroid eye disease evidenced by ocular examination and serological testing. He did have a strong family history of thyroid dysfunction, but he had never been diagnosed and the likelihood that his thyroid dysfunction could be advanced enough to cause compressive optic neuropathy yet evade detection is minimal. Neuro-imaging ruled out space-occupying lesions such as neuroblastomas and optic nerve gliomas.
3. Hereditary optic neuropathy
Leber hereditary optic neuropathy occurs due to mitochondrial DNA mutations. It often presents in the first or second decade of life, and is an important differential to rule out in a patient with bilateral optic neuritis. Typically there is disc hyperemia with blurred margins, and there may be dilated capillaries on the disc.2 The patient underwent genetic screening for Leber hereditary optic neuropathy, and his screening test was negative.
4. Optic neuritis
Pediatric optic neuritis is not a common condition; its incidence is less than 0.00005% annually.6-8 It is, however, an important condition to recognize and properly manage. Potentially life-threatening etiologies such as space-occupying lesions must be excluded initially with the use of neuro-imaging. A lumbar puncture with cerebrospinal fluid analysis can identify other serious etiologies of a swollen nerve, such as meningitis. This patient’s clinical findings of asymmetrically reduced visual acuity, an afferent pupillary defect, and the absence of a space-occupying intracranial lesion were indicative of bilateral papillitis.9 After excluding life-threatening diagnoses, the major categories of papillitis should be investigated: demyelinating, infectious, non-infectious and parainfectious.
a. Demyelinating
Demyelination may occur to the optic nerve sheath alone (isolated optic neuritis), or it may be a widespread, systemic phenomenon. Characteristic MRI findings of optic neuritis include foci of T2 bright signal, areas of enhancement within/surrounding the optic nerve, and possible optic nerve enlargement.3 Contrast-enhanced T1-weighted MR images have a high sensitivity for detecting acute optic neuritis and typically show enhancement of the inflamed portion of the optic nerve.3 Demyelinating optic neuritis neuro-imaging findings may be enhanced by the fluid-attenuated inversion recovery (FLAIR) pulse sequence. Optic neuritis may be the presenting sign of systemic disorders such as MS, acute disseminated encephalomyelitis (ADEM), or neuromyelitis optica (NMO). Although the AQP-4 serology test to rule out NMO was still in development at the time of the initial presentation of the patient in this case report, the patient had no systemic symptoms of spinal cord demyelination, which is necessary in the diagnosis of NMO. These disorders were ruled out by the aforementioned neuro-imaging; more advanced imaging of the spinal cord was likely not obtained due to the patient’s normal gait, unaffected motor coordination, and lack of other neurologic deficits. Despite the normal neuro-imaging, the patient may still be at heightened risk for future development of demyelinating disorders. The evidence is inconclusive regarding the long-term risk of such conditions following pediatric optic neuritis.10
b. Infectious
Bartonella henselae, the gram-negative bacillus responsible for cat-scratch disease, is capable of causing neuroretinitis in children more often than adults. Neuroretinitis is characterized by a swollen optic nerve and retinal exudates.9 According to the patient’s records, no exudates or hemorrhages were noted within the retina, thus greatly reducing the likelihood of neuroretinitis. Sinus-related optic neuritis is another cause of infectious optic neuritis, but is usually associated with a severe headache and spheno-ethmoidal sinusitis; our patient had neither of these findings.2 Syphilis may cause a unilateral or bilateral optic neuritis, but our patient was healthy and had no history of exposure to syphilis.2 Lyme disease, caused by the spirochete Borrelia burgdorferi, may cause optic nerve swelling. This patient had no history of a tick bite and his Lyme titer was negative.
c. Non-infectious
Papillitis or retrobulbar optic neuritis may occur in patients with sarcoid if the optic nerve is affected by granulomatous inflammation.3 Pain is often absent in this type of optic neuritis and a mild vitritis may be present.3 Sarcoid was not a likely differential in our patient due to his age and health history. Optic neuritis may also be present in patients with vasculitis such as systemic lupus erythematosus and polyarteritis nodosa. An antinuclear antibody screen was negative, making it highly unlikely that this patient’s optic neuritis was the result of a systemic vasculitis.
d. Parainfectious
Many viral illnesses such as measles, mumps, infectious mononucleosis, pertussis and chicken pox may result in a subsequent optic neuritis. Vaccinations may also cause optic neuritis by initiating an immune response that may ultimately target the optic nerve. This patient was diagnosed with optic neuritis secondary to vaccinations, a parainfectious cause of optic neuritis. The patient had no other predisposing factors to optic neuritis other than the possible association with the hepatitis B or tetanus vaccine he had received four weeks earlier. It is also plausible that the patient’s optic neuritis was idiopathic, rather than induced by the vaccine, because temporal association does not always indicate causation.
Differential diagnosis for optic atrophy pertinent to present-day examination
When the patient was first examined at the Eye Institute in 2014, he was asymptomatic, but objective measures such as the dilated fundus examination and OCT revealed optic atrophy. His optic atrophy was secondary to his history of optic neuritis as child, but if his history had been unremarkable, the potential list of differentials would be relatively brief. Given his age, primary open-angle glaucoma or congenital glaucoma would be extremely rare. There were no signs of secondary glaucoma. A hereditary optic atrophy would fit with the patient’s optic nerve appearance, but it is not consistent with the patient’s excellent visual function and lack of symptoms.
Clinical tests
When a pediatric patient presents acutely with a swollen optic nerve head and decreased vision, an MRI and lumbar puncture are necessary in order to exclude the most prognostically detrimental conditions. The MRI can detect conditions such as optic nerve gliomas, hydrocephalus and other space-occupying lesions. The lumbar puncture is important for identifying elevated intracranial pressure, and it can also aid in the diagnosis of meningitis, encephalitis and leukemia.
This patient’s 2014 exam was more than a decade after his optic neuritis had resolved, and the most ominous threat to vision was the development of optic atrophy. The patient presented to the clinic long after any treatable condition had subsided, and he did receive the most appropriate treatment at the time of his acute optic neuritis. The patient’s visual acuity was 20/15 OD, OS which indicated that the optic atrophy, though anatomically detectable, had not yet affected visual function to a large degree. In patients with a history of optic neuritis, it has been well-documented that although acuity returns to original levels, contrast sensitivity is impaired. In this patient, contrast sensitivity (1.95) was found to be normal according to the Pelli-Robson test. No relative afferent pupillary defect, a finding that would be present in both acute optic neuritis and asymmetric optic atrophy, was noted. A red cap test revealed no significant difference between the two eyes, an expected finding because the optic neuritis was largely symmetrical. Color vision was normal in both eyes. Upon dilated examination, the optic nerves did appear atrophic (Figures 1A and 1B). The optic nerve atrophy was also seen on OCT, which did indicate RNFL loss in both eyes (Figure 2). A Humphrey visual field test was performed to examine the correlation. It showed minimal diffuse loss on the mean deviation and no diffuse loss on the pattern deviation, demonstrating that the patient did not have functional vision loss (Figures 3A and 3B).
Clinical decision-making
Decision-making in this case centered on four major questions.
1. How did the patient’s history relate to the exam findings?
a. This patient presented with a history of one episode of optic neuritis 10 years prior. It was important to incorporate this known history with the ocular signs present during the exam, such as RNFL thinning and optic atrophy visible funduscopically. Normally, any type of RNFL thinning would be unexpected in a 23-year-old and an immediate cause of concern, but in this situation the cause was evident from the history. Discovering the likely etiology of this episode was important in determining the patient’s prognosis. For example, if the optic neuritis was secondary to a viral illness, the long-term prognosis is excellent without any intervention. If the neuropathy was associated with toxicity or malnutrition, the prognosis is less favorable.
i. An appropriate question is why the neuro-imaging was normal if the patient was diagnosed with optic neuritis. The imaging was ordered by an emergency department physician with no note of the slice thickness. It is possible the slices were too thick to observe inflammation if optic neuritis was not specifically considered in the emergency department. The imaging report specifically refers to ruling out the presence of a mass, so the focus of imaging may have been on too large a scale for this patient. This demonstrates the need for the optometric physician to continue to be involved in the imaging process.
b. The OCT (Figure 2) indicated pronounced thinning in all quadrants in both eyes. While this is unsettling, the anatomical changes have not yet manifested as functional visual field loss. This OCT is consistent with the expected findings for a patient with a history of bilateral optic neuritis.11 Due to technological advances such as OCT, subtle anatomical structural changes are detectable before visual function declines.12 This phenomenon explains why the OCT indicates thinning, but the visual field remains relatively normal with excellent visual acuity. It is also possible that the optic nerve is more resilient in childhood when this patient’s papillitis occurred, so the damage was milder than expected. Multiple studies have found that visual acuity and visual fields remain unaffected until pronounced thinning of the RNFL occurs.13-14 Contrast sensitivity testing is considered a more sensitive method than traditional Snellen visual acuity measurement for detecting small declines in visual function.15 A small study of RNFL thickness in patients with a history of optic neuritis found that for every line of decrease of contrast sensitivity, the mean RNFL thickness decreased by four microns.16
2. Are any threats to vision present?
a. The damage from the prior episode of optic neuritis was irreversible, but the patient should be carefully examined for any additional conditions that may pose a threat to vision. Initially the C/D ratio was judged as .2 OD/.3 OS without edema or pallor, but in the eyecare clinic, the nerves were assessed as .7 OD/.65 OS. Perhaps the difference between these two ratios was inter-observer variance, but it could also indicate that the nerve was swollen initially but it was missed because of the physiologically large C/D. Alternatively, RNFL rim thinning could have resulted from the initial presentation, but this is doubtful due to the largely intact visual field. When carefully examining the optic discs for edema, such as in this patient, it is important to assess the size of the optic nerve head. If the disc itself is small, the axons are crowded into a smaller space, mimicking edema. If the disc is large, the axons have a greater area to occupy, which could make the rim tissue appear thinned when it is not. This patient had true pallor secondary to his prior papillitis (evidenced by the OCT), but measuring the optic nerve head is a valuable exercise clinically because it gives a broader perspective on the health of the optic nerve. A recurrence of optic neuritis could certainly be considered a threat to vision due to its long-term effects on the optic nerve, but in this case recurrence is unlikely. It cannot be ruled out that the appearance of the optic nerve was due to some other pathological process, but this scenario is unlikely as Ockham’s razor proposes that the simplest explanation is often the most likely. Glaucoma could result in damage to the RNFL similar to that seen in this patient, but the likelihood of a healthy 23-year-old developing glaucoma with no other predisposing factors is very low. The most predictive signs of non-glaucomatous cupping are patient age less than 50 years and optic disc pallor in excess of cupping, which were consistent with the patient’s presentation.17,18 Other than continued RNFL loss with subsequent effects on the rim tissue of the optic nerve, no other threats to vision persist long-term in this patient.
3. Is there any treatable condition at this time?
a. The adage “treat the treatable” is applicable in this scenario. Optic atrophy is not treatable once the inciting agent has been removed. The argument could be made that this patient is more susceptible to any factor that might further damage the optic nerve, such as increased IOP, because the optic nerve is already compromised. It could be argued that treatment with ocular hypotensives should be initiated to protect the remaining RNFL, but the mechanism of damage has already been eliminated so there is no active process to treat. Treating a young, healthy, asymptomatic patient with mild optic atrophy with topical hypotensives for the rest of his life is an aggressive strategy that may provide no benefit to the patient. The patient’s IOP in the office was 17 mmHg OU. The benefits of lowering these normal pressures further is of questionable benefit. Pachymetry was not performed, but if this patient had thin corneas it could provide additional evidence to treat as thin corneas have been shown to be an independent risk factor for the development of glaucoma per the Ocular Hypertension Treatment Study.19
4. How should the patient be managed moving forward?
a. This patient is at a higher risk of developing MS due to his history of optic neuritis. He only had a single occurrence of optic neuritis, which followed the typical course, so his risk is likely lower than a patient who had suffered multiple bouts of optic neuritis. The patient would also likely have a higher risk for developing NMO if he had experienced multiple episodes of optic neuritis. NMO may cause an atypical optic neuritis that tends to affect contrast sensitivity more severely and result in greater thinning of the RNFL than a typical demyelinating optic neuritis.19
b. As there is no condition to actively treat, the emphasis should be on patient education and appropriate follow-up. Close inspection of the optic nerve is important to potentially detect any additional pathology unrelated to the papillitis that may develop. It could be argued that annual exams are the only necessary follow-up. It is also reasonable to advocate performing a visual field test, RNFL OCT, and stereoscopic fundus photography annually to more carefully monitor the patient for the development of any co-morbidities (such as glaucoma development) that may require more aggressive treatment relative to a patient with no optic nerve damage.
Literature review
Vaccine-induced optic neuritis is not a common entity, and its existence may even be questionable as often there is only a correlation between the administration of a vaccine and optic neuritis rather than a proven causation. To establish causality, ideally there should be a dose-response association, reports of adverse effects at multiple locations, or a proposed biological mechanism by which the reaction could occur, in addition to a temporal association.20 Reports of vaccine-induced optic neuritis usually include a presentation from days to four weeks following vaccination although there are some reports of optic neuritis up to six months following the influenza vaccine.3,20-22 The most common vaccine associations with optic neuritis are with the hepatitis B vaccine and the influenza vaccine.22 The first report of a demyelinating disorder associated with the hepatitis B vaccine was in 1983, at which time the hepatitis B vaccine was plasma-derived. The production of the hepatitis B vaccine was altered in 1987 when the recombinant form of the vaccine became available, and this is the vaccine type the patient in this case would have received. Recombinant vaccines are produced by inserting genes for a specific antigen into a suitable vector, which is commonly a virus with a low virulence. The benefit to a recombinant vaccine is the low risk of adverse events. Hepatitis B is currently the only recombinant vaccine widely available. The use of adjuvants could also be the mechanism by which vaccines generate immune-related disorders, but one of the most implicated adjuvants, thimerosal, has now been effectively eliminated from childhood vaccines as the maximum allowable concentration is 0.00002% .23
Although the evidence is far from conclusive, it is possible that if optic neuritis is indeed an uncommon adverse effect associated with vaccines, it likely only occurs in genetically susceptible individuals. Specifically HLA-DRB1 and HLA-DQB1 have been implicated in these cases.21 One large study conducted by Payne et al. refuted the association of several vaccines with optic neuritis. This matched case-control study involving 1,131 cases of optic neuritis found no statistically significant difference between optic neuritis and the following vaccines: anthrax, smallpox, hepatitis B or influenza.21
Pathophysiology
The optic nerve is divided into four anatomical sections as it travels posteriorly: intraocular, intraorbital, intracanalicular and intracranial. The intraocular portion of the optic nerve is affected in papillitis as optic nerve head swelling is visible with funduscopic evaluation. In retobulbar optic neuritis, the intraocular portion of the nerve is spared, but the posterior myelinated segments are affected.
As the most common cause for a swollen nerve in children is a preceding viral infection, it seems reasonable that vaccines can also cause the same condition by stimulating the immune system. Although the exact mechanism is unknown, it is believed that the peripheral activation of T-cells may cross the blood-brain barrier, resulting in a delayed type 4 hypersensitivity reaction. The myelin surrounding the optic nerve is damaged by this reaction, and eventually the axon may also be compromised — damage that is evident on OCT.10 Predictably, optic atrophy is quite common in pediatric optic neuritis, with up to 89% of patients showing evidence of atrophy.24 Interestingly, in the pediatric population the degree of optic atrophy does not always strongly correlate with the level of visual acuity loss. 25
Presentation
Optic neuritis in adults usually presents with unilaterally reduced vision, an APD on the affected side and a normal-appearing optic nerve head. In the Optic Neuritis Treatment Trial (ONTT), which enrolled 457 adult patients, the most common symptoms of optic neuritis were vision loss and eye pain. In this trial, the median visual acuity in the affected eye was 20/60 but ranged from 20/20 to light perception.26 Photopsias and reduced color vision were present in 30% and 88% of patients in the ONTT, respectively.26 Vision loss initially progressed but began improving after two weeks.
This differs from the classic presentation of optic neuritis in children, which is bilaterally reduced vision with swollen optic nerve heads. A small study found that 66% of optic neuritis was bilateral in children, and 64% had a swollen optic nerve head, while only 1/3 of adults present with a swollen optic nerve head.27 Initial visual acuity is more often profoundly depressed in children than in adults.28 Although rare, optic neuritis is more likely to be a neuro-retinitis in children than in adults in which the optic nerve head edema is accompanied by retinal exudates and/or retinal hemorrhages. A headache is usually associated with pediatric optic neuritis, another distinction from the most common presentation of optic neuritis in adults.
Treatment
No large-scale clinical trial has focused on optic neuritis in the pediatric population; therefore, evidence is largely limited to case reports, case studies or cohort studies.
The ONTT provides strong evidence regarding the treatment of optic neuritis in adults, but unfortunately this evidence may not extrapolate to children because no pediatric patients were included. Some small trials have indicated that a conservative approach of no treatment is best in pediatric cases, but others have found a benefit to intravenous methylprednisolone.29-30 If involvement is bilateral and visual acuity is dramatically reduced, treatment is more likely to be beneficial.29-30
Although there is a lack of strong evidence advocating for treatment of optic neuritis in children, treatment with intravenous methylprednisolone for three to five days is a common treatment. A course of oral steroids with taper is often prescribed following the IV administration to reduce the likelihood of recurrence.30 The treatment itself is the same in adults and children, but in children the duration of treatment is typically longer with a slower taper. 30 This treatment is somewhat controversial because optic neuritis is largely self-limiting, but steroids are more likely to be prescribed in bilateral cases with severe visual reduction.
Prognosis
The prognosis in cases of pediatric optic neuritis is quite favorable. In general, pediatric patients tend to recover the majority of their vision in weeks and often have no lingering symptoms even though imaging can detect lasting damage from the period of inflammation. In a study by Jo et al, 80% of patients recovered 20/40 vision or better over an average of 2.30 months.32 The average final acuity in this study was 20/25.32 One study suggested that unilateral involvement is associated with a better visual prognosis than bilateral involvement.27 In a long-term follow-up study of 39 children with optic neuritis, 77% had no additional episodes of optic neuritis and only six patients (15%) developed MS.32 In this same study, a surprising 55% of patients had a normal visual evoked potential despite a prior episode of optic neuritis. This finding may indicate that the optic nerve is more resilient in childhood than adulthood. As in adults, optic neuritis may be the first sign of MS, but the association is much weaker. Although the likelihood of developing MS following a single episode of optic neuritis approaches 40% in adults, in children the statistics are less reliable due to the lack of large-scale clinical trials. A meta-analysis found that 29% of children with isolated optic neuritis developed MS, but the range in children has been reported to be as low as 4% and as high as 43%.33-34 In a small study of 15 patients with optic neuritis, unilateral involvement and older age at presentation conferred a higher risk of developing MS.27 These findings, however, are in conflict with a larger study that included 36 patients and found those with bilateral involvement were more likely to develop MS and that age of presentation was not predictive of MS risk.35 Even though the exact risk of MS in these pediatric patients is unknown, it is important to obtain an MRI of the brain to rule out any white matter lesions. Normal neuro-imaging at the time of optic neuritis seems to be indicative of a reduced risk of MS. One study found that no pediatric patients developed MS who had normal neuro-imaging initially.35
The most common clinical findings after an acute episode of optic neuritis are optic atrophy, decreased color vision and altered pupillary cycling.36 A patient’s visual acuity may return to normal, but he or she may still perceive a subjective difference in quality of vision between the two eyes. Tests such as contrast sensitivity and color vision can help detect this subtle deficit in visual function of the affected eye.
Conclusion
Optic neuritis can have a variety of presentations and etiologies, but its hallmark is inflammation of the optic nerve. In this case report, a parainfectious etiology related to vaccinations was most likely responsible for the patient’s bilateral papillitis in childhood. Optic neuritis should be treated properly in its acute phase, and then patients should be monitored long-term for the development of optic atrophy and systemic demyelinating diseases such as MS and NMO.
References
1. Chan JW. Optic Nerve Disorders-Diagnosis and Management. New York: Springer Science + Business Media;2007. Chapter 1, Optic Neuritis.
2. Kanski JJ. Clinical Ophthalmology: A Systemic Approach. Sixth Edition. Philadelphia:Elsevier Limited; 2007. Chapter 21, Neuro-ophthalmology.
3. Miller NR, Newman NJ, editors. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. Sixth Edition. Philadelphia: Lippincott Williams and Wilkins; 2005. Chapter 6, Optic Neuritis
4. Grosberg BM, Solomon S, Lipton RB. Retinal Migraine. Current pain and headache reports. 2005:9(4):268-71.
5. Olesen J, Tfelt-Hansen P, Welch KM, et al. The Headaches. New York, NY: Raven Press Ltd; 1993:421-426.
6. Wakakura M, Ishikawa S, Oono S. Incidence of acute idiopathic optic neuritis and its therapy in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group. Nihon Ganka Gakkai Zasshi.1995;99:93-97.
7. Bojic L, Ivanisevic M, Sinicic A. The incidence of optic neuritis in Split-Dalmatia county, Croatia. Coll Antropol. 2004;28:343-347.
8. Kinnunen E. The incidence of optic neuritis and its prognosis for multiple sclerosis. Acta Neurol Scand.1983;68:371-377.
9. Brodsky MC. Pediatric Neuro-ophthalmology. Second Edition. New York: Springer Science + Business Media; 2010. Chapter 3, The Swollen Optic Disc in Childhood.
10. Perez-Cambrodi RJ, Cubillana A, Merino-Suarez ML, et al. Optic neuritis in pediatric population: A review in current tendencies of diagnosis and management. Journal of Optometry. 2014;7:125-130.
11. Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383-91.
12. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Progress in Retinal and Eye Research. 2007;26(6):688-710.
13. Costello W, Hodge Y, Pan I, et al. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Multiple Sclerosis. 2008;14(7):893-905.
14. Ajtony C, Balla Z, Somoskeoys S, et al. Relationship between visual field sensitivity and retinal nerve finer layer thickness as measured by OCT. Invest Ophthalmol Vis Sci. 2007;48:258-263.
15. Trobe JD, Beck RW, Moke DS, et al. Contrast sensitivity and other vision tests in the Optic Neuritis Treatment Trial. Am Journal of Ophthalmology. 1996;121(5):547-553.
16. Fischer JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324-332.
17. Trobe JD, Glaser JS, Cassady J, Herschler J, Anderson DR. Nonglaucomatous excavation of the optic disc. Arch Ophthalmol. 1980;98(6):1046-50.
18. Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999;14:2:95-108.
19. Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302-308.
20. Miravalle A, Biller J, Schnitzler E, Bonwit A. Neurological complications following vaccinations. Neurol Research. 2010;32(3):285-292.
21. Pillar GP, Toubi E. Vaccination-induced bilateral optic neuritis: rare but existing. IMAJ. 2012;14:690-691.
22. Payne DC, Rose CE, Kerrison J, et al. Anthrax vaccination and the risk of optic neuritis in the United States Military 1998-2003. Arch Neurol. 2006;63:871-875.
23. Bale JF. Neurologic complications of immunization. J Child Neurol. 2004;19:405-412.
24. Boomer J, Siatkowski M. Optic neuritis in adults and children. Seminars in Ophthalmology. 2003;18(4):174-180.
25. Taylor D, Cuendel F. Optic neuritis in childhood. In: Hess RF, Plant GT, eds. Optic Neuritis. Cambridge: Cambridge University Press. 1986;73-85.
26. Optic Neuritis Study Group. The clinical profile of optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1991;109:1673.
27. Morales DS, Siatkowski RM, Howard CW, et al. Optic neuritis in children. J Pediatr Ophthalmol Strabismus. 2000;37(5):254-259.
28. Boomer J, Siatkowski M. Optic neuritis in adults and children. Seminars in Ophthalmology. 2003;18(4):174-180.
29. Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953-1962.
30. Bonhomme GR, Mitchell EB. Treatment of pediatric optic neuritis. Curr Treat Options Neurol. 2012 Feb;14(1):93-102.
31. Jo DH, Kim SJ, Chae JH, et al. The clinical characteristics of optic neuritis in Korean children. Korean J Ophthalmol. 2011;25(2):116-120.
32. Kriss A, Francis DA, Cuendet F, et al. Recovery after optic neuritis in childhood. J Neurol Neurosurg Psychiatry. 1988;51:1253-1258.
33. Riikonen R, Donner M, Erkkilä H. Optic neuritis in children and its relationships to multiple sclerosis: a clinical study of 21 children. Dev Med Child Neurol. 1988;30:349-359. doi: 10.1111/j.1469-8749.1988.tb14560.x.
34. Waldman AT, Stull LB, Galetta SL, et al. Pediatric optic neuritis and risk of multiple sclerosis: meta-analysis of observational studies. J AAPOS. 2011;15(5);441-446.
35. Wilejto M, Shroff M, Buncic JR, et al. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67:258-262.
36. Kirkham TH, Coupland SG. Multiple regression analysis of diagnostic predictors in optic nerve disease. Can J Neurol Sci. 1981;8(1):67-72.