Planning Ahead for Corneal Epithelial Dystrophy: A Teaching Case Report
Allison Chinn, OD
Abstract
Epithelial basement membrane dystrophy (EBMD) is an anterior corneal dystrophy that can be asymptomatic or cause blurred vision, recurrent corneal erosion or both. Management of EBMD ranges from monitoring at annual exams to aggressive treatment. Recent developments allow clinicians to treat EBMD patients with a variety of in-office techniques. This case report discusses the presentation of EBMD and treatment for the various severities of this potentially sight-threatening condition.
Key Words: epithelial basement membrane dystrophy, recurrent corneal erosion, epithelial debridement, diamond burr polishing, phototherapeutic keratectomy, photorefractive keratectomy, anterior stromal puncture, alcohol delamination
Background
Epithelial basement membrane dystrophy (EBMD) is the most common corneal dystrophy seen in clinical practice.1-6 Its appearance varies, which leads to frequent misdiagnosis, but presentation most often includes dot-like epithelial opacities, whorl-like fingerprint lines and circumscribed gray map-like patterns.4 It is for this reason that EBMD is also referred to as a map-dot-fingerprint dystrophy. A consistent feature in all presentations is the formation of microcysts in the corneal epithelium with alterations in the basement membrane. Histology shows thickening of the basement membrane with fibrillary protein deposited between the basement membrane and Bowman’s layer. The absence of hemidesmosomes within the basal epithelial cells is responsible for faulty epithelial adhesion to the underlying basement membrane, which results in recurrent corneal erosion (RCE).1 Management of EBMD focuses on maintaining patient comfort and treating situational RCE. Typical initial onset of EBMD is in the second decade of life. Approximately 10% of patients develop RCE in the third decade, while the remainder do not develop symptoms associated with RCE.1,2
This teaching case report highlights diagnostic tools and appropriate management of the patient with EBMD, both symptomatic and asymptomatic. It is intended for third- and fourth-year optometry students actively involved in clinical patient care. As this condition is the most common corneal dystrophy encountered in clinical practice, a solid knowledge base about the condition and the appropriate steps for management and treatment is essential for the practicing optometrist in any clinical setting. This case can be used as a teaching tool in a didactic setting during anterior segment discussion, and it can be utilized in seminars focused on patient care in the clinical environment. The techniques discussed in this report can aid in familiarizing the new optometrist with methods used for diagnosing and managing patients with anterior segment abnormalities.
Student Discussion Guide
Case description
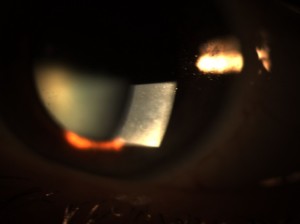
 Figure 1A and 1B. Diffuse subepithelial map-like geographic patterns consistent with corneal epithelial basement membrane dystrophy. |
A 51-year-old Caucasian female nurse practitioner presented to the clinic with complaints of blurred vision in both eyes at distance and near that had worsened over the past year. She reported that using reading glasses purchased over-the-counter provided relief. She also reported that her last eye exam was approximately one year ago. Her ocular history included a long-standing diagnosis of EBMD with no reported symptoms of RCE, and her medical history included genital herpes controlled with oral medication as needed. Family ocular history was positive for age-related macular degeneration (aunt and mother). Family medical history included pancreatic cancer (father), hypertension (mother), high cholesterol (sister) and stroke (maternal grandmother). The patient’s social history was positive for occasional alcohol consumption, and she denied tobacco or recreational drug use. Her blood pressure was 123/70 mmHg, right arm sitting at 4:18 p.m. Her height was 66 in., and her weight was 145 lbs. with a BMI of 23.4. Her medications included 2 mg lorazepam (Ativan) as needed for a sleep aid, and artificial tear supplements as needed. She reported medical allergies to celecoxib (Celebrex) and penicillins. She was oriented to time, place and person, and her mood was appropriate.
Entering uncorrected visual acuity was OD 20/30-2 distance and 20/100 near, and OS 20/30+1 distance and 20/80 near. Pupils were equal, round, reactive to light, with no signs of an afferent pupillary defect. Extraocular motilities exhibited full range of motion OU. Cover test revealed orthophoria at distance and 4-prism-diopter exophoria at near. Confrontation visual fields were full to finger-counting in each eye. Vision was correctable to 20/20 in each eye at distance and at near with manifest refractions of OD +1.00 DS, and OS +1.00-0.25×070 with 1.50 near add. Intraocular pressures measured with Goldmann applanation tonometry were within normal range, 11 mmHg OD and 10 mmHg OS at 4:18 p.m.
Slit lamp biomicroscopy of the anterior segment exam revealed normal adnexa, lids, lashes, puncta and bulbar and palpebral conjunctiva in both eyes. EBMD was confirmed in both eyes by the observation of diffusely scattered subepithelial map-like geographic patterns (Figure 1A and 1B). There was no evidence of ocular surface disruption with fluorescein dye and no sign of previous or current corneal erosion. Anterior chambers were deep and quiet without evidence of cells or flare. Chamber angles were 1:1/2 nasally and temporally using the van Herick method. Pupils were dilated using 1 drop 1% tropicamide and 1 drop 2.5% phenylephrine in each eye. Examination of the posterior segment was unremarkable: clear vitreous OU, clear crystalline lens OU, flat macula OU, attached peripheral retina OU, normal vasculature OU and optic nerve cup-to-disc asymmetry (0.60/0.60 OD and 0.45/0.45 OS), which was noted to be long-standing.
Because the patient’s vision was correctable to 20/20 in each eye, recommendations for treatment to control her fluctuating blurry vision were progressive addition lenses for full-time wear and tear supplements as needed. This presentation represents a typical EBMD clinical scenario.
Educational Guidelines
The following includes discussion points and a review of the literature to help facilitate discussion of the case and methods of managing EBMD. Additional information regarding pathophysiology and clinical presentation of EBMD is also included to further educate the clinician about the condition.
Learning objectives
At the conclusion of this case discussion, students should be able to:
1) recognize the signs and symptoms of epithelial basement membrane dystrophy
2) be familiar with the differential diagnosis associated with anterior corneal dystrophies
3) understand the histological process that results in epithelial basement membrane dystrophy
4) educate the patient about the condition, associated symptoms and treatment options
5) be familiar with in-office management techniques and recognize when surgical techniques are more beneficial for relief of symptoms
Key concepts
1) recognition of clinical signs and reported symptoms associated with epithelial basement membrane dystrophy
2) the importance of knowing current methods and indications for treatment of epithelial basement membrane dystrophy and its complications
Discussion points
1) Knowledge of corneal epithelial dystrophies
• identify the corneal epithelial dystrophies
• describe basic clinical signs of each epithelial dystrophy
• what symptoms are associated with patients with epithelial basement membrane dystrophy?
• describe the various clinical presentations of epithelial basement membrane dystrophy
• discuss the structural abnormalities of a cornea affected by epithelial basement membrane dystrophy
• describe the symptoms associated with recurrent corneal erosion
2) Clinical management and treatment
• discuss the appropriate clinical management of the asymptomatic and symptomatic patient with epithelial basement membrane dystrophy
• discuss the indications for procedural intervention for the symptomatic patient
• describe each method of treatment and compare the contraindications and advantages associated with each
• discuss the methods of treatment that can be performed by the primary care optometrist in a typical clinical setting
3) Patient education
• what pertinent information should the optometrist discuss with the patient?
• discuss educating the patient about the methods of treatment
• propose a treatment plan for the symptomatic patient with epithelial basement membrane dystrophy
4) Critical thinking
• in the absence of spectral domain optical coherence tomography, what clinical techniques can be used to aid in the diagnosis of epithelial basement membrane dystrophy?
• discuss a treatment plan for a noncompliant patient with recurrent corneal erosion and extensive epithelial damage and associated visual impairment
• discuss questions a patient may have when first diagnosed with epithelial basement membrane dystrophy
Discussion
Pathophysiology
EBMD is characterized by bilateral and frequently asymmetric subepithelial fingerprint lines, geographic map-like lines and epithelial microcysts. Clinically, there are at least three (or any combination thereof) epithelial configurations that may be observed: 1) groups of tiny, round or comma-shaped, grayish-white superficial epithelial opacities of various sizes in the pupillary zones of one or both eyes; 2) a fingerprint pattern of translucent lines best seen with retroillumination; and 3) a map-like or geographic pattern best seen on oblique illumination.7 A thickened basement membrane is one of the most important features of this condition, and it is caused by abnormal epithelium turnover, maturation and production of the basement membrane that leads the basal epithelial cells to extend superficially into the epithelium.6 Histology shows thickening of the basement membrane with deposition of fibrillary protein between the basement membrane and Bowman’s layer.1 Histologically, there are also corresponding patterns to the grayish dots, fingerprint pattern and map-like pattern observed with biomicroscopy. The grayish dots represent small cystoid spaces in the epithelium into which other superficial corneal epithelial cells desquamate. The fingerprint pattern is formed by both normally positioned and inverted basal epithelial cells producing abnormally large quantities of basement membrane. The map pattern is produced beneath the epithelium by basal epithelial cells and keratocytes that have migrated from the superficial stroma to elaborate both multilaminar basement membrane and collagenous material.7 The absence of hemidesmosomes of the basal epithelial cells can be responsible for the typical RCE.1 EBMD typically presents during the second decade of life, and RCE tends to present during the third decade. Although presentation is most commonly sporadic, EBMD can present with an autosomal dominant method of inheritance.1,2,6,7 It has been considered to be an age-dependent degeneration of the cornea.1,6 As was the case with this patient, EBMD generally is asymptomatic. Approximately 10% of patients will develop RCE, and many will manifest visually significant epithelial irregularity resulting in irregular astigmatism.1,2,6
RCE has an unknown pathophysiology, but the underlying etiology is the presence of abnormal corneal epithelial basement membrane adherence to Bowman’s layer, whether by abnormal adhesion complexes or a reduplication of the basement membrane itself.8 Various hypotheses exist to explain the defective adhesion of the epithelium to the underlying basement membrane: abnormality of the basement membrane, absent or abnormal hemidesmosomes, or increased activity of matrix metalloproteinases (MMP), especially MMP-2 and MMP-9.9
While EBMD is the most common corneal dystrophy encountered in clinical practice, it is important to be aware of other dystrophies that can affect the corneal epithelium and other layers of the cornea. This discussion focuses on the anterior corneal dystrophies that target the epithelium, including Meesmann, Lisch and Reis-Bückler’s dystrophies. Meesmann dystrophy is a rare, nonprogressive epithelial dystrophy that is observed during the first years of life but generally remains asymptomatic until middle age. Retroillumination reveals tiny intraepithelial cysts of uniform size but variable density throughout the cornea, usually centrally concentrated extending out toward but never reaching the limbus. Treatment for Meesmann dystrophy is usually not required, but a bandage soft contact lens or superficial keratectomy may be beneficial if photophobia is present or if visual acuity is severely affected.1,2 Lisch epithelial dystrophy was originally thought to be a variant of Meesmann, but is now believed to be a genetically distinct condition. Gray bands with a whorled configuration are observed during slit lamp examination, and retroillumination shows densely packed microcysts scattered diffusely across the cornea.1 Reis-Bückler’s epithelial dystrophy presents with subepithelial gray reticular or polygonal opacities that are seen primarily in the central cornea. Corneal sensation is decreased, and visual impairment may occur secondary to scarring of Bowman’s layer. Patients with Reis-Bückler’s epithelial dystrophy suffer from severe episodes of recurrent erosion that require treatment and may ultimately require corneal transplantation, but the dystrophy often recurs in the graft.1,2
Diagnosis
Diagnosing EBMD can be challenging given its variable appearance. Most diagnoses can be made by careful patient history and slit lamp examination, but there are techniques available for confirming or ruling out a potential case. Patients may describe a constant foreign body sensation, recurrent eye pain upon awakening, decreased vision, monocular diplopia, or shadow images. Frequency and severity of these symptoms can indicate an irregularity of the corneal epithelium. Vigilant slit lamp examination will reveal the typical signs associated with EBMD, and the clinician can observe the diffuse gray map-like patches, white dots or fine refractile fingerprint lines in the corneal epithelium. These findings can be seen best with retroillumination or a broad slit-lamp beam angled from the side.2 Performing retroillumination while the patient is dilated may also highlight additional corneal irregularities that may have been too subtle to notice with a broad beam. Negative fluorescein staining defects are also observed in patients with EBMD. The elevations in the ocular surface associated with EBMD result in an immediate tear film break-up over the corresponding area.10 Positive fluorescein staining is observed when a recurrent corneal erosion is present.
In vivo confocal microscopy has proven to be a helpful tool for examining the morphologic anomalies associated with EBMD, especially when the features are atypical. Corneal confocal microscopy can provide a qualitative morphological description and it can quantify pathology, making it useful for detection and management of pathologic and infectious conditions, detection and management of corneal dystrophies and ectasias, monitoring contact lens-induced changes, and pre- and post-surgical evaluations. The magnification and resolution provided by confocal microscopy allows for an extremely detailed evaluation of the corneal layers when suspected defects are not visible at the slit lamp.11 This technique does require direct contact with the cornea, and it could inadvertently cause more damage to the anterior surface.
The recent development of spectral-domain optical coherence tomography (SDOCT) has dramatically improved imaging not only for the retina but for the cornea as well. SDOCT can provide valuable diagnostic information when a corneal abnormality is suspected.6 According to a study conducted by Sanharawi et al. to determine the features of EBMD and the reliability of SDOCT in evaluating it, eyes with the condition demonstrated an irregular, thickened basement membrane with greater hyper-reflectivity when compared with the epithelial basement membrane in a normal control eye. The thickened epithelial basement membrane was sometimes compromised with the appearance of small hyper-reflective elevations associated with a protrusion of the basement membrane into the corneal epithelial layer. These protrusions into the epithelium usually corresponded to the map-like or fingerprint lesions observed during slit lamp examination.6 Another striking feature observed in SDOCT scans of patients with EBMD was the presence of hyper-reflective dots, which are thought to be epithelial cysts, beneath the abnormal epithelial basement membrane. In cases with normal basement membranes, the cysts were observed to be more superficial, but in cases where an abnormal basement membrane protrusion was found, the dots were always beneath the abnormal epithelial basement membrane. It is believed that maturing epithelial cells migrating from the deeper layers to the more superficial layers of the epithelium become trapped beneath the abnormal epithelial basement membrane and are prevented from surfacing and discharging from the corneal surface.12 The cells can then become vacuolated and liquefied to form the intra-epithelial cysts seen on slit lamp exam and SDOCT scans.12
Sanharawi and colleagues also noted separation between the corneal epithelial layer and Bowman’s layer in patients with a history of RCE. The epithelial detachments corresponded to the map-like lesions. All scans were repeated to determine the reproducibility and repeatability of this technique. Agreement between two corneal specialist observers was perfect for all SDOCT features with the exception of detection of a thickened basement membrane for which agreement was substantial but not perfect. Additionally, SDOCT results were compared to in vivo confocal microscopy evaluations and found to be as reliable and much less invasive in the diagnosis of EBMD.6
Treatment
Treatment for EBMD focuses on maintaining patient comfort and treating situational RCE. Approximately 10% of patients will develop RCE, and the remainder of patients will not develop symptoms.1 Patient education regarding the basic pathophysiology of EBMD and RCE is important for managing the condition and symptoms appropriately. Patients should have a clear understanding of the condition itself, symptoms to be alert for, how EBMD could potentially affect vision, and the different modes of treatment available for both EBMD and situational RCE.
The treatment of RCE can include a cycloplegic drop for pain management, a prophylactic antibiotic solution/ointment 4-6 times daily, and 5% sodium chloride hypertonicity ophthalmic ointment (Muro 128) 4 times daily. After the epithelial defect has healed, artificial tears and bland ointments are recommended along with Muro 128 ointment for at least 3-6 months to prevent recurrence. In the absence of an ointment, an adjunctive bandage contact lens with the topical cycloplegic/prophylactic antibiotic solution have proven effective in some cases.2 A recent development in treatment for RCE is the application of autologous serum eye drops. These drops administered to treat ocular surface disease often produce better results than antibiotics, corticosteroids or tear supplements.13 Autologous serum therapy is considered effective for treating ocular surface disease because fibronectin within the autologous serum is thought to promote epithelial migration and anchorage. Additional growth factors and anti-inflammatory mediators provide additional comfort and potential long-term relief for the patient.14
Medical therapy has also been found effective compared with standard lubrication therapies to reduce symptoms and frequency of RCE. Oral doxycycline and topical corticosteroids, alone or in combination, have proven beneficial for managing RCE by inhibiting extracellular matrix degradation by matrix metalloproteinases.8,15 Doxycycline inhibits MMP-9 and also exhibits properties thought to facilitate lipases from bacteria present on lid margins, which ultimately improves meibomian gland dysfunction and leads to stable tear film quality.8
If corneal erosions persist, surgical intervention is indicated. The two most commonly employed procedures for management of patients with significant corneal epithelial irregularity associated with EBMD are epithelial debridement with diamond burr polishing of Bowman’s layer (ED+DBP) and phototherapeutic keratectomy (PTK).16 ED with diamond burr polishing of Bowman’s layer is especially common for larger defects and for defects along the visual axis.2 It is typically performed at the slit lamp with topical anesthesia and placement of an eyelid speculum. A cellulose sponge or blunt spatula is used to debride 7-10 mm of central corneal epithelium, and then a hand-held battery-driven diamond burr is used to gently and uniformly polish Bowman’s membrane in the entire area of epithelial defect in a vertical fashion for approximately 10 seconds.9 A bandage soft contact lens is placed on the treated eye and removed following resolution of the epithelial defect, and prophylactic antibiotic drops are given 4 times a day for 1 week.9 Several studies have indicated that ED+DBP is superior to ED alone because it may be associated with a decreased risk of future development of RCE and recurrent EBMD.16
Results have been mixed regarding long-term effectiveness of ED alone and ED+DBP. Whereas both procedures produce a statistically significant improvement in best-corrected visual acuity, Itty et al. reviewed results using ED alone and found that approximately one quarter of the treated eyes developed recurrent dystrophic epithelial disruption over an average follow-up period of 33 months.17 Tzelikis et al. examined the results of ED+DBP and reported that none of the treated eyes demonstrated recurrent epithelial changes over an average follow-up of 22 months.18 Aldave and coworkers performed a retrospective case series study and concluded that ED+DBP should be considered the procedure of choice as it eliminated RCE in 96% of treated eyes and successfully treated visually significant epithelial irregularity in 100% of treated eyes in this series. Postoperative complications from ED are minor but can include photophobia, foreign body sensation, spontaneous corneal erosion, persistent epithelial irregularity or subepithelial haze. Visually significant recurrences are uncommon, but repeat ED can provide a successful outcome.17
While PTK is also an effective treatment for the management of both RCE and visually significant epithelial irregularity, ED+DBP is a more convenient treatment option because it can be performed at the slit lamp or in a minor procedure room without the need for access to an excimer laser.9,16 PTK and ED are similarly effective. PTK uses an excimer laser to ablate the superficial stroma and simultaneously remove the abnormal epithelium, allowing a potentially more stable epithelium to regenerate.2,19 In treating the stroma and Bowman’s layer, a new bed for the migrating epithelium cells is formed, the anterior stroma is stimulated to form new anchoring fibrils, and, consequently, an improved hemidesmosome adhesion can be formed.20 In a retrospective case review comparing PTK and ED+DBP, Sridhar et al. found that both groups obtained symptomatic relief; however, patients treated with ED+DBP had a lower incidence of postoperative haze and a lower recurrence rate.21
While ED+DBP and PTK are the most common procedures employed to treat EBMD and RCE, additional treatment options include surface ablation, anterior stromal puncture (ASP), and alcohol delamination of epithelium. For the visually symptomatic patient with EBMD, photorefractive keratectomy (PRK) is the procedure of choice to treat refractive error, while PTK may be performed to treat RCE or irregular astigmatism.19 PRK may have an additional therapeutic effect due to the removal of abnormal epithelium. PRK was identified as a safer alternative for the correction of refractive error when compared to laser-assisted in situ keratomileusis (LASIK) because the faulty attachments between the epithelial basement membrane and Bowman’s layer make for an unstable corneal surface that is susceptible to sloughing during LASIK. For this reason, LASIK is contraindicated in patients with EBMD because they are predisposed to epithelial ingrowth, flap melting, flap distortion and exacerbation of symptoms.21 PRK in combination with PTK is a safe and reliable treatment for the correction of refractive error and alleviation of symptoms associated with EBMD.21
ASP can be performed with a needle or with neodymium-yttrium aluminum garnet (Nd:YAG) laser. ASP is effective for treating RCE because it prevents erosions by inducing fibrosis that causes epithelium to adhere tightly to the underlying basement membrane.9 Although it is a recognized treatment for managing RCE, it is generally not a treatment option for visually significant epithelial irregularity because it is associated with a greater risk of permanent corneal scarring.2,9,16 ASP is generally used in symptomatic, refractory cases, and most often reserved for traumatic erosions with focal areas of abnormal epithelium outside of the visual axis as the scars it will induce can cause visual disturbances.2,9
Two other in-office treatments, alcohol delamination and topical cocaine, have been proven effective recently for EBMD. During alcohol delamination, the cornea is swabbed with alcohol and thoroughly washed, and the affected epithelium is peeled loose. Afterwards, an unpreserved antibiotic drop is given, and a bandage contact lens is applied until the epithelial defect has resolved.22,23 Sayegh et al. treated symptomatic EBMD patients with 4% topical cocaine followed by epithelial debridement and achieved results comparable with studies using ED+DBP and PTK.24 Their results indicated a significant improvement in mean visual acuity, a total recurrence rate of 9%, and a rate of recurrences that needed subsequent intervention of 3%. Topical cocaine acts as an effective topical anesthetic and, because of its adrenergic effect, causes vasoconstriction that retards its own absorption. It enables an approximately 20-minute anesthetic effect. Cocaine likely acts similarly to alcohol by cleaving the anchoring fibrils between Bowman’s layer and the corneal epithelial basement membrane, removing the abnormal basement membrane, including any sub-basal cellular debris, and leaving behind a smooth surface that enables a firmer adhesion of new epithelial cells.23,24
Conclusion
This teaching case report describes the management of symptomatic and asymptomatic EBMD. Diagnosis based on an attentive case history and astute slit lamp examination is critical for appropriate treatment. Fortunately, there are several methods of treatment for the complication of RCE in patients who are burdened with EBMD. It is important to remember that while only 10% of patients with EBMD will present with clinical RCE complaints, this population of patients can have morphologic characteristics that can induce significant visual impairment. It is essential for the primary eyecare provider to understand the etiology of EBMD and the various management options available in order to provide the most appropriate treatment.
Acknowledgements
I extend my special thanks to Janene Sims, OD, PhD, FAAO, and Elizabeth Steele, OD, FAAO, for offering their time and professional critiques through review of this case report. I also wish to express great appreciation to Caroline Pate, OD, FAAO, for her constant motivation, encouragement and support throughout my residency.
References
1. Kanski J, Bowling B. Clinical ophthalmology: a systematic approach. 7th ed. China: Elsevier Limited; 2011.
2. Ehlers J, Shah C. The Wills Eye Manual: office and emergency room diagnosis and treatment of eye disease. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008.
3. Webvision: The Organization of the Retina and Visual System; Map-Dot Fingerprint Dystrophy [Internet]. Webvision; c2015 [cited 2015 April 4]. Available from: https://webvision.med.utah.edu/2012/04/map-dot-fingerprint-dystrophy/.
4. Veire E. IC3D: Classifying Corneal Dystrophies [Internet]. Review of Cornea and Contact Lens; c2010 [cited 2015 April 4]. Available from: https://www.reviewofcontactlenses.com/content/d/disease/c/21310.
5. Hereditary Ocular Disease: Corneal Dystrophy, EBMD [Internet]. The University of Arizona: Arizona Board of Regents; c2015 [cited 2015 April 4]. Available from: https://disorders.eyes.arizona.edu/category/alternate-names/ebmd.
6. Sanharawi ME, Sandali O, Basli E, et al. Fourier-domain optical coherence tomography imaging in corneal epithelial basement membrane dystrophy: a structural analysis. Am J Ophthalmol. 2015;159(4):755-763.
7. Yanoff M, Fine BS. Ocular pathology. 5th ed. St. Louis, MO: Mosby, Inc.; 2002.
8. Mark E, Hammersmith KM. Review of diagnosis and management of recurrent erosion syndrome. Current Opinion in Ophthalmology. 2009;20(4):287-291.
9. Suri K, Kosker M, Duman F, et al. Demographic patterns and treatment outcomes of patients with recurrent corneal erosions related to trauma and epithelial and Bowman layer disorders. Am J Ophthalmol. 2013;156(6):1082-1087.
10. Ramsey AC. Vital Stains: What You Really Need to Know [Internet]. Review of Cornea & Contact Lenses; c2011 [cited 2015 September 24]. Available from: https://www.reviewofcontactlenses.com/content/d/irregular_cornea/c/27820/.
11. Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008; 2(2):435-445.
12. Waring GO III, Rodrigues MM, Laibson PR. Corneal dystrophies. I. dystrophies of the epithelium, Bowman’s layer, and stroma. Surv Ophthalmol. 1978;23(2):71-122.
13. Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye & Contact Lens: Science & Clinical Practice. 2015;41(3):133-140.
14. Kronemyer B. Autologous serum drops relieve dry eye over long term [Internet]. Ocular Surgery News: Cornea/External Disease; c2015 [cited 2015 April 4]. Available from: https://www.healio.com/ophthalmology/cornea-external-disease/news/print/ocular-surgery-news/%7B21800779-6871-4a21-9f16-bcd1297c64af%7D/autologous-serum-drops-relieve-dry-eye-over-long-term.
15. Mencucci R, Favuzza E. Management of recurrent corneal erosion: are we getting better? Br J Ophthalmol. 2014;98:150-151.
16. Aldave AJ, Kamal KM, Vo RC, Fei Y. Epithelial debridement and Bowman’s layer polishing for visually significant epithelial irregularity and recurrent corneal erosions. Cornea Clinical Science. 2009;28(10):1085-1090.
17. Itty S, Hamilton SS, Baratz KH, Diehl NN, Maguire LJ. Outcomes of epithelial debridement for anterior basement membrane dystrophy. Am J Ophthalmol. 2007;144(2):217-221.
18. Tzelikis PF, Rapuano CJ, Hammersmith KM, Laibson PR, Cohen EJ. Diamond burr treatment of poor vision from anterior basement membrane dystrophy. Am J Ophthalmol. 2005;140(2):308-310.
19. Woreta FA, Davis GW, Bower KS. LASIK and surface ablation in corneal dystrophies. Surv Ophthalmol. 2015;60(2):115-122.
20. Dedes W, Faes L, Schipper I, Bachmann LM, Thiel MA. Phototherapeutic keratectomy (PTK) for treatment of recurrent corneal erosion: correlation between etiology and prognosis – prospective longitudinal study. Graefes Arch Clin Exp Ophthalmol. 2015;253(10):1745-9.
21. Sridhar MS, Rapuano CJ, Cosar CB, Cohen EJ, Laibson PR. Phototherapeutic keratectomy versus diamond burr polishing of Bowman’s membrane in the treatment of recurrent corneal erosions associated with anterior basement membrane dystrophy. J Ophthalmol. 2002;109(4):674-679.
22. Chan E, Jhanji V, Constantinou M, et al. A randomised controlled trial of alcohol delamination and phototherapeutic keratectomy for the treatment of recurrent corneal erosion syndrome. Br J Ophthalmol. 2014;98(2):166-71.
23. Dua HS, Lagnado R, Raj D, et al. Alcohol delamination of the corneal epithelium: an alternative in the management of recurrent corneal erosions. J Ophthalmol. 2006;113(3):404-411.
24. Sayegh RR, Kouyoumjian PB, Vedula GG, et al. Cocaine-assisted epithelial debridement for the treatment of anterior basement membrane dystrophy. Cornea. 2013;32(6):889-892.