Plateau Iris Syndrome and Acute Angle Closure Glaucoma: A Teaching Case Report
Theresa Zerilli, OD, FAAO, Tam Nguyen, OD, MS, FAAO, Christine L. Burke, OD, FAAO, and Jonathan R. Hamilton, OD, FAAO
Abstract
Plateau iris syndrome (PIS) is one cause of acute angle closure glaucoma. An anteriorly rotated or abnormally oversized ciliary body occludes the ciliary sulcus, blocking access to the trabecular meshwork and resulting in elevated intraocular pressure. Iridotomy is performed to relieve pupillary block. Management options include topical medications, iridoplasty, cycloplasty and cataract extraction. This article documents a case of PIS highlighting the pathogenesis, diagnosis and management of this rare condition.
Key Words: plateau iris configuration, plateau height, complete and incomplete plateau iris syndrome, acute angle closure glaucoma.
Background
Primary angle closure glaucoma is characterized by apposition of the peripheral iris to the trabecular meshwork as a result of abnormal size and position of anterior segment structures or posterior segment pressure forces that alter the anterior segment anatomy.1,2,3 The four most common causes of angle closure glaucoma are: pupillary block, plateau iris, lens-induced angle closure and ciliary block.2 Plateau iris syndrome (PIS) is the most common etiology of angle closure in relatively young patients, while in the older population, pupillary block accounts for almost all cases.4 A structural anomaly in which the pars plicata is anteriorly displaced or abnormally large is responsible for the plateau iris.2
PIS is also to be differentiated from plateau iris configuration (PIC). Whereas PIC refers to a normal anterior chamber depth, flat iris plane, but narrow angle on gonioscopy before surgical treatment, PIS is a diagnosis that refers to the potential for an angle to occlude in patients with PIC in the presence of a patent iridectomy or iridotomy.5
PIS is further classified into two forms: complete and incomplete. They are differentiated by the height of the plateau, which refers to the level of the iris surface in relation to the angle structures. In the complete form, the height of the plateau is such that it closes the anterior and posterior trabecular meshwork, resulting in no drainage and subsequent rise in intraocular pressure (IOP).2,6,7 In contrast, the incomplete form does not result in IOP rise because partial closure allows for adequate drainage.
It is paramount for the astute clinician to not only identify the mechanism of acute angle closure but also to be proficient in methods of analyzing the iridocorneal angle with gonioscopy, ultrasound biomicroscopy (UBM) and optical coherence tomography (OCT) so that timely treatment and management can be initiated. Furthermore, PIS should be considered as a causative factor in patients with IOP rise following laser peripheral iridectomy or iridotomy.
This teaching case report is intended for optometry students at all levels. For first- and second-year students, emphasis can be placed on the anatomical structures of the iridocorneal angle, mechanisms of acute angle closure, diagnostic testing that can be performed and the mechanisms of action for medications used in treatment. For third- and fourth-year students and residents, emphasis can be placed on treatment and management of cases with persistent angle closure and glaucoma despite laser treatment.
Case Description
A 56-year-old Caucasian male presented for a comprehensive eye exam without any visual complaints. His last eye examination had been 15 months prior, and he reported an unremarkable exam at that time. The patient stated that he had a history of “narrow angle attacks,” which had resolved after bilateral laser peripheral iridotomies (LPIs) 13 years prior. The patient denied any history of glaucoma or being treated with topical medications. Family ocular history revealed that his maternal grandmother had been diagnosed and treated for glaucoma. The patient’s medical history was remarkable for multiple sclerosis (MS) and hyperlipidemia. His current medications included interferon beta-1a (Avonex) and simvastatin daily. At his last visit with neurology, the patient was noted to have clinically stable MS on the current regimem of Avonex. He had no reported allergies to medications. The patient stated that he had never been a smoker, did not drink and did not use recreational drugs. He was oriented to time, person and place and his mood and affect were appropriate.
The patient’s entering best-corrected visual acuity was 20/20 OD and OS at distance and at near. Pupils were equally round and reactive to light without an afferent defect in either eye. Extraocular muscles were unrestricted and full in all fields of gaze. Confrontational visual fields were full to finger counting in both eyes. Color vision was full in each eye as measured with Ishihara plates. Manifest subjective refraction was OD +7.25 -2.25 x 100 OD and OS +5.00-2.25 x 075 with 20/20 visual acuity at distance in each eye. Anterior segment examination by slit lamp revealed bilaterally patent LPIs, a white and quiet conjunctiva with clear corneal layers OD and trace pigment on the central corneal endothelium OS. The irises were both flat. The anterior chamber was normal depth and without cells or flare. There were early nuclear sclerotic lens changes in both eyes. Intraocular pressures as measured by Goldmann tonometry were 14 mmHg OD and 34 mmHg OS at 10 a.m. Sussman 4-mirror gonioscopy showed the deepest structure seen OD to be posterior trabecular meshwork in all clock hours and anterior trabecular meshwork from 10 o’clock to 2 o’clock. In the left eye, the deepest structure seen was the posterior trabecular meshwork for 240 degrees, except at 10 o’clock where iris-corneal touch was noted. The deepest structure in the remaining 120 degrees was anterior trabecular meshwork. The patient refused a dilated fundus examination despite education on the importance and the need for assessment of sight-threatening disease. Undilated fundus exam was performed using a 78D Volk lens and revealed shallow, healthy pink rim tissue without pallor OU. The cup to disc ratio was assessed as 0.35 round OD and 0.45 round OS. The maculae were clear and flat with no pathology noted.
On that same day, the patient was evaluated by the glaucoma specialist. The specialist concluded that the increase IOP OS was most likely a consequence of damage to the trabecular meshwork from past episodes of acute angle closure prior to having undergone LPI’s in each eye. He supported this tentative diagnosis by noting that the patient was asymptomatic, presented with no signs of inflammation, had patent LPIs, and did not have peripheral anterior synechiae on gonioscopy. The specialist recommended having the patient return in one month for an IOP test to establish a diurnal IOP curve, dilated fundus examination, pachymetry and a 24-2 SITA-standard Humphrey visual field test.
Follow-up 1: three weeks after initial presentation
The patient returned to the eye clinic three weeks later to complete the glaucoma workup as recommended by the specialist. At this visit, however, the patient reported an episode of unilateral left eye pain with associated blurred vision. He also stated that the pain lasted for two hours. The patient then admitted that these same symptoms had been occurring over the past year at a frequency of one episode every few weeks. He noted that the visual symptoms were also occasionally accompanied by a headache and had the propensity to occur in the late afternoon or evening. At this exam, presenting visual acuities with glasses were 20/25 OD and OS. Pupils, extraocular muscle testing, confrontational visual fields and slit lamp examination were all stable since the last visit. Intraocular pressures by Goldmann tonometry were recorded as 14 mmHg OD and 19 mmHg OS at 9:10 a.m. Intraocular pressures were retaken at 10:30 a.m. and were noted as 15 mmHg OD and 20 mmHg. Pachymetry revealed central corneal thickness to be 522 microns OD and 558 microns OS. The patient was dilated using 1% tropicamide and 2.5% phenylephrine OU. Dilated fundus examination revealed early nuclear sclerotic lens changes in both eyes. The cup to disc ratio was 0.35 round OD and 0.45H/0.40V OS, without pallor OU. Temporal and inferior-temporal thinning was noted on the optic disc OS. All other aspects of the posterior segment were unremarkable. Humphrey visual field testing showed no defects OD and an early superior arcuate defect OS, which was consistent with the thinning that was previously noted on the neuroretinal rim OS. Post-dilation IOPs by Goldmann tonometry, taken at 11:45 a.m. were 15 mmHg OD and 34 mmHg OS. At that time, one drop of 0.5% apraclonidine hydrochloride (Iopidine) was instilled in the left eye. Post-dilation, Sussman 4-mirror gonioscopy showed the deepest structure in the right eye to be the posterior trabecular meshwork for 270 degrees and anterior trabecular meshwork for the remaining temporal 90 degrees. In the left eye, the deepest structure was anterior trabecular meshwork for 270 degrees with no structures visible in the remaining temporal 90 degrees. Repeat tonometry by the same method used previously was 12 mmHg OD and 18 mmHg OS. At the conclusion of the exam, the assessment was likely narrow angle glaucoma OS and the patient was started on 0.004% travoprost (Travatan) qhs OS only. He was scheduled for a follow-up appointment in six weeks.
Follow-up 2: nine weeks after initial presentation
The patient returned for his second follow-up visit for an IOP check to determine the efficacy of the treatment initiated at the previous visit. He reported good compliance on the current regimen of travoprost 0.004% qhs OS. When questioned about recurrent instances of intermittent blurred and painful vision, the patient noted only having one episode since his last eye exam. He felt that his vision was stable and denied any other ocular complaints. The entrance examination findings were stable with prior exams and tonometry via Goldmann was 16 mmHg OD and 15 mmHg OS at 10 a.m. The patient was instructed to continue with the current regimen and to return to the clinic in two to three months for a follow-up appointment, or sooner if painful or blurry vision occurred.
Follow-up 3: 17 weeks after initial presentation
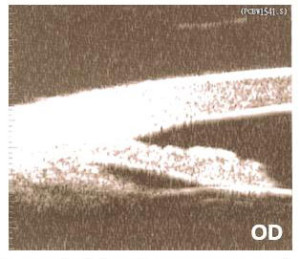
Figure 1. Ultrasound biomicroscopy of the right eye.
Click to enlarge
The patient returned to clinic two months later reporting more frequent episodes of blurred vision accompanied by periorbital pain OS. He noted that the symptoms were lasting up to three hours and felt that they were now occurring two to three times per week. The patient also realized that these symptoms typically occurred after waking in the morning or after taking a nap. He noticed that these episodes mimicked the symptoms he had experienced prior to having LPIs. The patient reported compliance with the 0.004% travoprost qhs OS. Visual acuities, pupils, extraocular motilities, confrontation fields, color vision and slit lamp examination were again stable. IOPs via Goldmann tonometry were recorded as 11 mmHg OD and 11 mmHg OS at 2 p.m. Gonioscopy was repeated at this exam with careful attention to the iris approach into the angle. Sussman 4-mirror gonioscopy revealed a flat iris approach centrally with a steep approach into the angle OU. The deepest structure visible OD was the scleral spur in all clock hours. The deepest structure visible OS was the anterior trabecular meshwork except from 3-9 o’clock where appositional touch was apparent. Indentation gonioscopy revealed posterior trabecular meshwork. The patient was diagnosed with complete PIS OS and was referred to a tertiary center for UBM.

Figure 2. Ultrasound biomicroscopy of the left eye following argon laser peripheral iridoplasty.
Click to enlarge
UBM confirmed PIC OD and complete PIS OS. The patient was treated with argon laser peripheral iridoplasty (ALPI) OS the same day following UBM. (Figures 1 and 2) The patient was scheduled for a follow-up appointment in two weeks and told to continue with the current medication, 0.004% travoprost qhs OS.
Follow-up 4: 19 weeks after initial presentation
The patient presented to this exam reporting one episode of painful, blurry and cloudy vision when he woke from a nap. The symptoms lasted three to four hours before completely resolving. He felt the symptoms were slightly less intense than prior to having ALPI OS. The patient confirmed good compliance with the current medication, travoprost qhs OS. Again, all entrance testing and slit lamp evaluations yielded normal findings. IOPs via Goldmann tonometry were 15 mmHg OD and 16 mmHg OS at 11:39 a.m. Repeat gonioscopy did not reveal a widening of the angle. The same structures were seen OS as noted prior to ALPI. The patient was scheduled to return to the clinic in two weeks for evaluation with a glaucoma specialist.
Follow-up 5: 21 weeks after initial presentation
The patient returned to the clinic as scheduled but presented to the visit with an injected left eye in conjunction with a mid-dilated pupil. When questioned he reported having four episodes of pain with blurring of vision in that eye since his last visit. The most recent episode lasted 10-12 hours, and his wife noted that his pupil was larger in the left eye. He reported that the symptoms were mitigated after going into the bright sunlight. The patient also noted strict adherence to administering 0.004% travoprost qhs OS. Visual acuity was stable at 20/20 OD and OS. Pupils were not equal. In dim illumination, the pupils were measured at 4.0 mm OD and 6.0 mm OS, and in bright illumination, 3.0 mm OD and 5.5 mm OS. Both eyes were round and reactive to light, but OD was more brisk with a grade 3+ response vs. a grade 1 response OS. No afferent pupillary defect was noted. Extraocular motilities and confrontational visual fields were full OU. Slit lamp examination was stable from last exam OD but revealed perilimbal flush OS. The anterior chamber was without cells and flare and the LPIs remained patent OU. IOPs measured by Goldmann tonometry were recorded as 14 mmHg OD and 51 mmHg OS at 8:43 a.m. Sussman 4-mirror gonioscopy OS showed anterior trabecular meshwork from 4-8 o’clock and no other structures in the remaining clock hours with appositional touch. Indentation gonioscopy again opened the angle to anterior trabecular meshwork. The glaucoma specialist started the patient on a 500 mg tablet of acetazolamide (Diamox) once daily, dorzolamide hydrochloride/timolol maleate (Cosopt) bid OS only, and pilocarpine 2% qid OS only. The 0.004% travoprost qhs OS was discontinued. The patient was followed closely and a repeat IOP check at 1 p.m. revealed that IOP in the left eye had dropped to 6 mmHg. The patient remained on Diamox for four more days and then the acetazolamide was discontinued. The glaucoma specialist recommended the patient remain on the current regimen of dorzolamide hydrochloride/timolol maleate bid OS and pilocarpine 2% qid OS until his next follow-up appointment.
Follow-up 6: 22 weeks after initial presentation
The patient returned to the eye clinic one week later for follow- up. He denied any new episodes of painful, blurry vision OS and reported 100% compliance with the current medication regimen of dorzolamide hydrochloride/timolol maleate bid OS and 2% pilocarpine qid OS. Visual acuity was stable at 20/20 OD and OS. Pupils were not equal secondary to induced miosis OS from the pilocarpine. All other entrance tests as well as slit lamp evaluation were unremarkable. IOP was measured at 17 mmHg OD and 11 mmHg OS at 11:08 am with Goldmann tonometry. Sussman 4-mirror gonioscopy was performed OS and no structures were seen in the nasal clock hours. With compression technique, the deepest structure seen was posterior trabecular meshwork in that quadrant. In the remaining quadrants, the deepest structure seen was the anterior trabecular meshwork. The patient was told to continue his current regimen of dorzolamide hydrochloride/timolol maleate bid OS and 2% pilocarpine qid OS and was scheduled to return to the glaucoma specialist in two weeks.
Follow-up 7: 24 weeks after initial presentation

Figure 3. Gonioscopy following argon laser peripheral iridoplasty in our patient. The white arrow denotes the incorrectly placed mid-peripheral laser burn.
Click to enlarge
The patient was seen two weeks later by the glaucoma specialist. The patient reported compliance with the current regimen of dorzolamide hydrochloride/timolol maleate bid OS and 2% pilocarpine qid OS and denied any episodes of painful, blurry vision. At this visit, visual acuity was stable at 20/20 OD and OS. Pupil findings were stable; however, the pupils again were not equal secondary to the effects of pilocarpine. All other findings were also stable. IOP by Goldmann tonometry was measured at 14 mmHg OD and 12 mmHg OS at 11:15 a.m. Gonioscopy was repeated and graded the same as during the previous exam. The glaucoma specialist recommended that the patient remain on the current medical regimen of dorzolamide hydrochloride/timolol maleate bid OS and 2% pilocarpine qid OS. The specialist noted that if episodes were to recur, ALPI would be repeated. Furthermore, he noted that the recent ALPI was not successful because the placement of the laser was not as peripheral as recommended (Figure 3) and the burns were not sufficiently intense to render effective contraction and subsequent opening of the angle. He recommended that the patient be monitored closely with repeated gonioscopy, and ultimately cataract extraction, when there was a secondary decrease in visual acuity. The patient remained on dorzolamide hydrochloride/timolol maleate bid OS and 2% pilocarpine qid OS and denied any episodes of painful, blurry vision at his three- and six-month follow-up appointments.
Key Concepts
- Recognizing the importance of probing further into a patient’s past ocular history to help guide the clinician to the correct diagnosis
- Recognition of the signs and symptoms of angle closure glaucoma and plateau iris syndrome
- Recognition of available ancillary testing that may lead to the correct diagnosis
- Understanding that treatment is not based solely on the identification of acute angle closure glaucoma, rather, it is driven by mechanisms responsible for angle closure
- The importance of post-treatment and management of patients with acute angle closure
Learning Objectives
- To understand the signs and symptoms of acute angle closure glaucoma
- To understand the ocular anatomy and the mechanisms of acute angle closure glaucoma
- To gain knowledge with regard to the differential diagnosis in patients with persistent angle closure
- To understand the condition of angle closure glaucoma, specifically plateau iris syndrome, and recognize when appropriate ancillary diagnostic testing is necessary
- To understand the typical patient demographic of the disease profile
- To provide proper patient education with regard to expectations, treatment options, importance of ancillary testing and follow-up appointments
- To understand the optometrist’s role in the workup, management and co-management of a patient with this condition
- To understand the treatment options for a patient with this condition and refer for treatment in a timely manner in order to prevent ocular morbidity
Discussion Questions
1. Knowledge and concepts required for critical review of the case
a. What are the anatomical differences between normal ocular structures, PIS and PIC?
b. What is the difference between complete and incomplete PIS?
c. What tests during the examination would be helpful in the diagnosis of the case?
d. What ancillary testing would be helpful in the diagnosis of the case?
2. Differential diagnosis
a. What are the ocular differential diagnoses in a patient with persistent symptoms despite treatment?
b. How can the list of differentials be narrowed?
c. Which of the differentials made the most sense with this history?
3. Disease management
a. How do we treat this condition in order to minimize ocular morbidity?
b. How is a patient like this managed after treatment has been initiated?
c. When is treatment usually initiated?
d. What topical medications are used in the treatment of angle closure glaucoma?
e. What is the mechanism of action for each class of these topical medications?
f. What ancillary surgical treatment can be used in the treatment of angle closure glaucoma and what is the mechanism of action?
4. Patient education
a. What do we tell the patient about his or her disease and long-term prognosis?
b. What symptoms do we tell the patient to look out for?
c. How do we properly educate this patient to understand the importance of regular follow-up care?
5. Critical thinking
a. What were the complicating factors in this case?
b. How would you have managed this case differently?
Learning Assessment
- Clinical skills such as gonioscopy, tonometry, anterior segment OCT and UBM may be tested in a proficiency clinical exam
- Knowledge base of the condition can be tested via diagrams and photos of normal and abnormal gonioscopy findings
- Knowledge base of the condition can be tested via anterior segment OCT test results of normal vs. PIC
- Knowledge base of the condition can be tested using normal vs. PIC UBM findings
- Clinical thinking skills can be assessed by case reports that are either hypothetical examples or from a review of the literature
Discussion
Epidemiology
Angle closure glaucoma is a relatively rare occurrence in Caucasians and is found in approximately 2% of this population.8 The occurrence of angle closure in young Caucasians is even more infrequent. PIS is the most common etiology of angle closure in relatively young patients, while in the older population pupillary block accounts for almost all cases.4 Ritch et al. studied the prevalence of PIS in patients 40 years or younger with angle closure and found that 54% of this population had PIS.9 Similarly, Stieger et al. found the occurrence of PIS in their studied population of patients 60 years or younger with angle closure to be 47%.1 Another large retrospective study showed that the mean age of diagnosis for patients with PIS was 35.9 Evidence also suggests that this anatomical phenomenon is familial following an autosomal dominant inheritance pattern.10
Pathophysiology and mechanisms of angle closure
The four mechanisms of angle closure are pupillary block, plateau iris, lens-induced, and angle closure originating posteriorly to the lens/iris diaphragm. The latter includes ciliary or malignant block from aqueous misdirection. Pupillary block occurs when aqueous humor cannot pass from the posterior to the anterior chamber because of iridolenticular apposition. Plateau iris occurs when an anteriorly rotated or abnormally large ciliary body occludes the ciliary sulcus, blocking access to the trabecular meshwork and resulting in elevated IOP. Angle closure can also occur if a cataractous lens pushes the central and posterior lens forward, causing iridotrabecular apposition. Similarly, ciliary block or aqueous misdirection also causes iridotrabecular apposition where interaction between the lens capsule and the anterior vitreous obstructs aqueous flow from the ciliary processes to the anterior chamber.11
Differentials for persistent angle closure and PIS in our patient
Given the persistence of self-reported, recurrent episodes of intermittent, painful and blurry vision with concurrent moderate rises in IOP measured in-office, the differential diagnoses were revisited. The initial diagnosis considered at the patient’s first visit was prior damage to the trabecular meshwork secondary to past episodes of acute angle closure attacks prior to LPIs. This mechanism, however, would lead to a chronic elevation without the large fluctuations in IOP seen at follow-up visits. Another differential considered was pigment liberation, post-dilation, given the rise in IOP recorded after the patient was dilated at his second visit. Meticulous gonioscopy and slit lamp evaluation of the anterior chamber post-dilation did not support this theory. Two other differential diagnoses that warrant exclusion in a patient with narrow angles status post-LPIs are re-closure of the LPIs and persistent peripheral anterior synechiae. Slit Lamp evaluation confirmed patency of the apertures and gonioscopy verified the absence of synechiae, ruling these out as potential causes. The patient’s past history of acute angle closure and subsequent bilaterally patent LPIs removed all question of pupillary block as the mechanism of angle closure. Furthermore, slit lamp exam revealed an incipient cataract, noncontributory to angle closure. Likewise, the lack of recent intraocular surgery without evidence of consistent elevated IOP and no signs of a shallow central anterior chamber on slit lamp exam provided low suspicion for ciliary block as the mechanism of angle closure. A last differential is pseudo plateau iris caused by iris or ciliary body cysts, but this was not consistent with the patient’s UBM or gonioscopy findings. Thus, complete PIS was the most likely cause of angle closure in this patient.
Diagnosis of acute angle closure and PIS
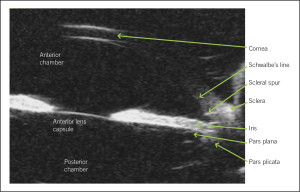
Figure 4. Ultrasound biomicroscopy (UBM) of a normal eye. This is a typical cross-sectional, in vivo image that can be obtained using UBM. The anatomic relationships between the structures can be evaluated easily.
Click to enlarge
The method of choice for diagnosing PIS is UBM (Figure 4) because of its high resolution images of the region, including the ciliary body and the posterior chamber.12 Anterior segment OCT is useful in identifying characteristics of narrow angles and can also quantitatively measure the anterior chamber angle. It is less invasive than UBM as it does not require topical anesthesia or direct contact with the ocular surface. However, OCT is inferior to UBM as it does not detail the ciliary body positioning and the iridociliary relationship. Lastly, it goes without saying that gonioscopy is a very important test. In our patient, it was used to determine angle closure and to rule out pigment liberation post-dilation and peripheral anterior synechiae. On gonioscopy, the angle is found to be narrow, and there is a drop-off of the peripheral iris. Furthermore, investigators found that the “double hump” or “sine wave” sign detected by indentation gonioscopy can indicate the presence of plateau iris, even in the presence of a patent iridotomy.13 On the iris, the peripheral hump is caused by the ciliary body propping up the iris root, whereas the central hump represents the central third of the iris resting over the surface of the lens.4 It is thought that compression forces the aqueous humor behind the iris, causing the double hump sign pre- and post-laser treatment.13 Performing gonioscopy in the fellow eye can also provide useful clues because angle closure glaucoma is often bilateral and asymmetric.11
Treatment and management of PIS
For cases of acute angle closure glaucoma, treatment is often initiated before cause is determined because of the detrimental effects of prolonged IOP elevation on ocular structures. Typically, topical aqueous suppressants including beta- blockers, alpha-agonists and carbonic anhydrase inhibitors are used. Prostaglandin analogs can be used despite a common perception that this class of medication takes longer to work.11 Pilocarpine, a direct-acting cholinergic, can also be used. In addition to its miotic effects, mechanically pulling the iris away from the trabecular meshwork, it also stimulates contraction of the ciliary muscle, thereby increasing trabecular outflow of aqueous humor. Pilocarpine is also effective at lowering IOP as it promotes peripheral iris thinning, opening the anterior chamber angle.14 Pupillary constriction, induced myopia, brow ache and retinal detachment are potential side effects of this medication. Oral or intravenous acetazolamide 500 mg can be used for IOP greater than 40 mmHg. Intravenous mannitol can be used in cases where IOP is greater than 50-60 mmHg or if the earlier treatments are ineffective after two hours.11 Following control of acute IOP elevation, the next step is to perform iridectomy or iridotomy to eliminate pupillary block as the cause. The next treatment option to consider is APLI, which has also been proposed as a definitive treatment for PIS.15,16 During this procedure, large-diameter contraction burns tighten the peripheral iris and cause thinning to the peripheral iris stroma, preventing angle closure upon dilation. In a few studies, APLI has been shown to be effective and safe.15,16 In the case described here, APLI was not effective for two reasons. First, the burns created by the laser were not of adequate intensity to cause effective contraction. Second, it was also proposed that the location of the burns was not peripheral enough to cause effective contraction. Similarly, one review did not find strong evidence for the use of APLI in this subset of angle closure patients.17
Lens extraction is not a standard treatment for PIS; however, it can be an option when the lens plays a role in obstruction of the angle. Anterior displacement of the anterior lens capsule with age can cause changes in the central or axial anterior chamber depth and raise the height of the plateau and narrow the angle. In the current case, although the mechanism of angle closure was PIS, cataract extraction was considered after ineffective APLI treatment because it lowers the overall iris plateau height. Ultimately, when surgical intervention is refused or surgical treatment has failed, patients are placed on maintenance doses of pilocarpine. Unfortunately, very few studies have evaluated the exact dose and duration necessary to mitigate acute attacks.
In our case, visual field testing showed no defects OD and an early superior arcuate defect OS, which was consistent with the thinning that was previously noted on the neuroretinal rim OS. Of course it is paramount that management of these patients include regular visual field testing to asses functional damage and adjust intraocular target pressures as necessary.
Conclusion
This teaching case report discusses the treatment and management of a patient with acute angle closure glaucoma secondary to complete PIS. Recognition of PIS as a cause in the development of primary angle closure is critical. Following laser peripheral iridotomy, the patient must continue to be monitored for recurrent angle closure with careful gonioscopy as well as regular visual fields. The eyecare provider should be able to identify the clinical characteristics of plateau iris syndrome in order to prevent incorrect diagnosis, which can lead to the development of peripheral anterior synechiae, chronic angle closure or acute angle closure.
References
1. Stieger R, Kniestedt C, Sutter F, Bachmann LM, Stuermer J. Prevalence of plateau iris syndrome in young patients with recurrent angle closure. Clin Experiment Ophthalmol. 2007;35(5):409-13.
2. Shield MB. Textbook of glaucoma 2nd ed. Baltimore, MD: Lipincott Williams & Wilkins, 1987.
3. Chang BM, Liebmann JM, Ritch R. Angle closure in younger patients. Trans Am Ophthalmol Soc. 2002;100:201-12.
4. Ritch R, Dorairaj S. Plateau iris syndrome in younger patients. Clin Experiment Ophthalmol. 2007;35(5):399-400.
5. Wand M, Grant WM, Simmons RJ, Hutchinson BT. Plateau iris syndrome. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(1):122-30.
6. Wand M, Pavlin CJ, Foster FS. Plateau iris syndrome: ultrasound biomicroscopic and histologic study. Ophthalmic Surg. 1993;24(2):129-31.
7. Canals M, Costa-Vila J, Potau JM, Merindano MD, Ruano D. Scanning electron microscopy of the human zonule of the lens. Acta Anat. 1996;157(4):309-14.
8. Bankes JL, Perkins ES, Tsolakis S, Wright JE. Bedford glaucoma survey. Br Med J. 1968;1(5595):791-6.
9. Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology. 2003;110(10):1880-9.
10. Etter JR, Affel EL, Rhee DJ. High prevalence of plateau iris configuration in family members of patients with plateau iris syndrome. J Glaucoma. 2006;15(5):394-8.
11. Rosenblum H, Radcliffe N. Case-based approach to managing angle closure glaucoma with anterior segment imaging. Can J Ophthalmol. 2014;49(6):512-8.
12. Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113(4):390-5.
13. Kiuchi Y, Kanamoto T, Nakamura T. Double hump in indentation gonioscopy is correlated with presence of plateau iris configuration regardless of patent iridotomy. J Glaucoma. 2009;18(2):161-4.
14. Pavlin CJ, Foster FS. Plateau iris syndrome: changes in angle opening associated with dark, light, and pilocarpine administration. Am J Ophthalmol. 1999;128(3):288-91.
15. Lai JS, Tham CC, Chua JK, Lam DS. Efficacy and safety of inferior 180 degrees goniosynechialysis followed by diode laser peripheral iridoplasty in the treatment of chronic angle-closure glaucoma. J Glaucoma. 2000;9(5):388-91.
16. Ritch R, Tham CC, Lam DS. Argon laser peripheral iridoplasty (ALPI): an update. Surv Ophthalmol. 2007;52(3):279-88.
17. Ng WS, Ang GS, Azuara- Blanco A. Laser peripheral iridoplasty for angle closure. Cochrane Database Syst Rev. 2008;16(3):CD006746.




